Biofilms are organized communities of microorganisms that exist in virtually every natural environment. Biofilms form in response to shear force (flow) to avert being removed from their environment by “turning on” specific genes responsible for biofilm growth.
Biofilm formation is a process involving several steps:
- migration,
- attachment,
- layering and
- mucopolysaccharide (slime) formation.
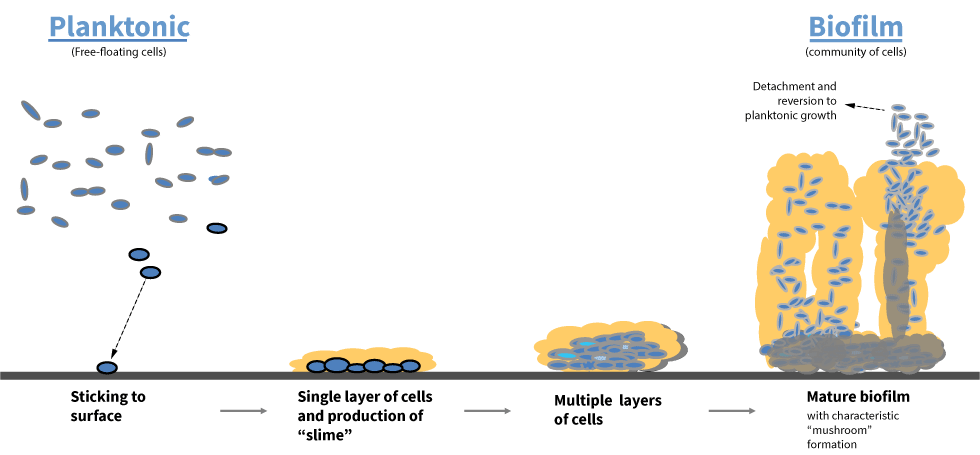
Some of these steps involve chemical messengers, known as quorum-sensing compounds, which form a rudimentary communication system among microorganisms.
Once formed, microbial and bacterial biofilms are difficult to remove as they show an inherent lack of susceptibility to biocides and antibiotics when compared to planktonic (free-floating) microorganisms. Many studies have shown greater than a thousand-fold resistance to antibiotics by biofilms when compared to the same bacteria in a planktonic state.
When contemplating situations where bacterial biofilms would be expected to grow, it becomes obvious why they are so common. The two requirements for growth, 1) presence of microorganisms and 2) presence of flow, covers a multitude of animal, plant and industrial situations in the world. The simplest examples of bacterial biofilms are the slippery coating on stones in a stream, and the plaque that forms on human teeth. However, the medical and industrial impact of biofilms is tremendous and costly.
At Innovotech we know the science of biofilms. It’s in our DNA. Our contract research staff are available to assist you in fighting biofilm formation on your products.
Antibiotic, Antimicrobial, and Biocide Resistance
The Problem
In a biofilm state, bacteria are typically less susceptible to antibiotics, antimicrobials, and biocides. In some cases, bacteria can be up to 4,000 times more resistant than the same organism in a free-floating state. Comparisons of MIC (minimum inhibitory concentration) tests with MBEC (minimum biofilm eradication) tests vividly display the differences in susceptibility from the planktonic to the biofilm state. In fact, using an MIC test in evaluation of what treatments to use against a biofilm infection can inadvertently result in treating the biofilm with sublethal concentrations of antibiotics, thereby increasing the chances of development of resistance.
Claims of microbial resistance therefore must be segregated into 2 components: genetic resistance (genes turned on by the organism that produces factors involved in protecting the organism) and phenotypic resistance (the construction of the organism itself i.e. cell walls and mucosal protection).
70% of bacteria causing hospital-based infections are resistant to at least one of the antibiotics used to treat them. More than 2 million patients in the US each year contract an infection as a result of receiving healthcare in a hospital, with 90,000 cases resulting in death.
Innovotech’s Solution
The first step of a solution is therefore to conduct sensitivity testing using the organism in a biofilm state. Only Innovotech technology can conduct this testing in a high-throughput fashion. The most prevalent organisms in microbial and bacterial biofilm infections are Pseudomonas sp., Staphylococcus sp., Streptococcus sp., and many fungi.
Specific diseases include:
| Pseudomonas sp | Staphylococcus sp | Streptococcus sp | Fungi |
|---|---|---|---|
|
|
|
|
The second step is to recognize that it is unlikely that a single antibiotic will be effective against a biofilm owing to its inherent increased resistance and therefore, at the very least, an approach that incorporates both single and combination antibiotics is required (Biofilm susceptibility to metal toxicity). Again, Innovotech technology allows testing in a high throughput fashion saving both time and expense over other tests.
Testing can be conducted by Innovotech through Innovotech’s contract research unit or, if the client has suitable microbiology and biofilm knowledge, the MBEC Assay® Kit can be purchased (click here to “Order”).
http://www.astm.org/Standards/E2799.htm
The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms.
Biofilm bacteria: formation and comparative susceptibility to antibiotics.
The Problem with Implanted Medical Devices
Innovotech has a full understanding of the various implanted medical device testing procedures available. Our experience has helped us streamline and problem solve many of the issues that can delay testing.
Implanted medical devices (joints, catheters, stents, pacemakers, etc.) provide a solid surface for microbial growth upon which biofilms quickly form. A typical biofilm infection can cost as much as US$47,000 per patient to treat, and costs can escalate with chronic and recurrent infections.
Biofilms cause over 2 million infections annually, resulting in an estimated US$11B in additional patient healthcare costs. Hospital-acquired infections cost US$1.8B annually with urinary catheter use associated with up to 90% of these infections. Implanted medical device testing can reduce these costs.
Innovotech’s Solution to Implanted Medical Device Testing
Innovotech’s proprietary technology and industry expertise provide medical device manufacturers with an accepted approach to first evaluate antimicrobial/anti-adherence coatings to study their impact on biofilm-forming microorganisms, and then develop research studies to validate product efficacy claims suitable for regulatory submission.
Our antimicrobial research team has worked with clients to achieve 510K approval in less than 12 months, owing to the high throughput nature of the company’s in vitro technology.
